Potentiometric titration end point detection and indicators
Many things that have been told about use of indicators in acid-base titration hold also for potentiometric titrations. The higher the concentration of the titrated substance and the titrant, the longer the steep part of the titration curve and the easier the redox indicator selection. In the case of one color indicators, potential at which indicator color starts to be visible depends on the indicator concentration. Depending on the situation we should either titrate to the full change of color or to the first visible change of color - and so on.
However, there are also important differences. The most obvious one is - while the general idea that observed color depends on the ratio of concentrations of both reduced and oxidized forms still holds, ratio of concentrations is not pH dependent, but redox potential dependent. We can easily calculate ratio of the concentrations of both forms using Nernst equation:
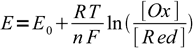 1
1Let's assume - as we did in the case of pH indicators - that for the complete color change we have to move from 10:1 to 1:10 concentration ratio. That means we have to move from the potential
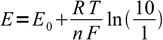 2
2to
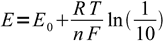 3
3or by
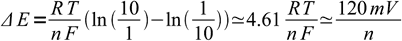 4
4at 25 °C (more precisely it should be 118.2 mV, but as we started with an approximate rule 10:1 to 1:10, such accuracy is not necessary). This is a useful rule of thumb - 120 mV will be enough always. For many indicators reaction requires 2 electrons, so 60 mV change is enough for the observable color change.
Table below contains some of the popular redox indicators. Note, that reduced forms of many indicators are colorless - that means, that indicator concentration plays important role. Also note, that many of these substances are weak acids/bases, thus formal potentials of their reactions can depend on the solution pH. Some of these substances are even used as pH indicators, so their color depends both on the pH and redox potential of the system, which makes selecting them even more complicated.
| indicator name | reduced form color | oxidized form color | normal potential at pH 0 (V) | normal potential at pH 7 (V) |
|---|---|---|---|---|
| Safranin T | colorless | red | 0.24 | -0.29 |
| Neutral red | colorless | red | 0.24 | -0.33 |
| Indigomonosulfonic acid | colorless | blue | 0.26 | -0.16 |
| Phenosafranin | colorless | red | 0.28 | -0.25 |
| Indigotetrasulfonic acid | colorless | blue | 0.36 | -0.05 |
| Methylene blue | colorless | green-blue | 0.36 | ??? |
| Nile blue | colorless | blue | 0.41 | ??? |
| Benzidine | colorless | blue | 0.92 | ??? |
| Variamine blue B | colorless | blue | 0.69 | ??? |
| Diphenylbenzidine | colorless | violet | 0.76 | TS |
| Diphenylamine sulfonic acid | colorless | red-violet | 0.85 | TS |
| Erioglaucine A | green | red | 1.00 | ??? |
| p-Ethoxychrysoidine | red | light yellow | 1.00 | TS |
| Setoglaucine | yellow-green | light red | 1.06 | ??? |
| p-Nitrodiphenylamine | colorless | violet | 1.06 | ??? |
| Ferroin | red | light blue | 1.06 | ??? |
| 5-Nitroferroin | red-violet | light blue | 1.25 | ??? |
| 2,2'-Bipyridine (Ru complex) | colorless | yellow | 1.33 | TS |
TS - the same, redox potential is not pH dependent. ??? - at this point we were not able to locate information that we trust.
Interestingly, in the case of three popular potentiometric titrations we usually don't use redox indicators, but specific indicators, that work only in the case of these methods.
In the case of permanganometry there is no need for indicator - small excess of permanganate is immediately visible, as the permanganate itself has a very strong color. As we need some excess of the titrant, it makes sense to start with a blank test, to check what volume of excess titrant has to be added before the color change can be spotted.
In the case of iodometric titration, we use starch. Free iodine adsorbs at the starch surface, changing its color to blue. Depending on the titration type (and titrant) starch will either allow determination of the first traces of excess iodine, or determination of the moment when last traces of iodine disappear. In the latter case it is important to add starch close to the endpoint, as product of the iodine-starch reaction created when iodine concentration is high is relatively stable. Iodine itself is colored and its solutions are yellow, but intensity of the color is usually too low to be useful for endpoint detection.
In the case of bromine titration we can use methyl orange as an indicator - once the excess free bromine appears in the solution, it will oxidize the indicator and solution turns colorless. This is an example of application of irreversible redox indicator.
If you want to select an indicator for your method, you can try approach similar to that described in the acid-base titration end point detection section - calculate redox potential of your system for 99.9% and 100.1% titration and choose an indicator that changes color between these values.




